Popular Posts
-
General Chemistry
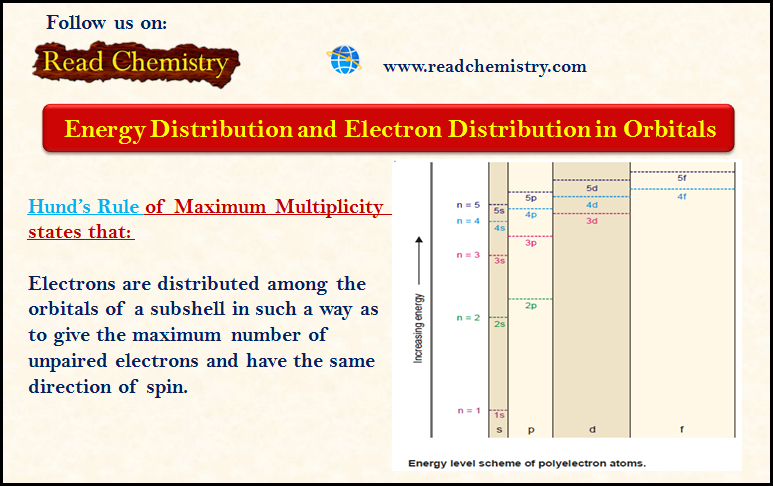
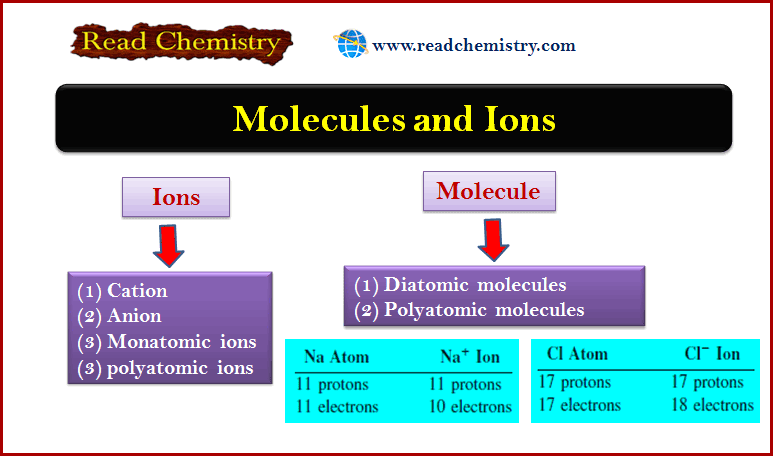
Distribution of Electrons in Orbitals
– In this subject, we will discuss the Distribution of Electrons in Orbitals according to Hund’s Rule. Energy Distribution and…
Read More » -
Organic Chemistry
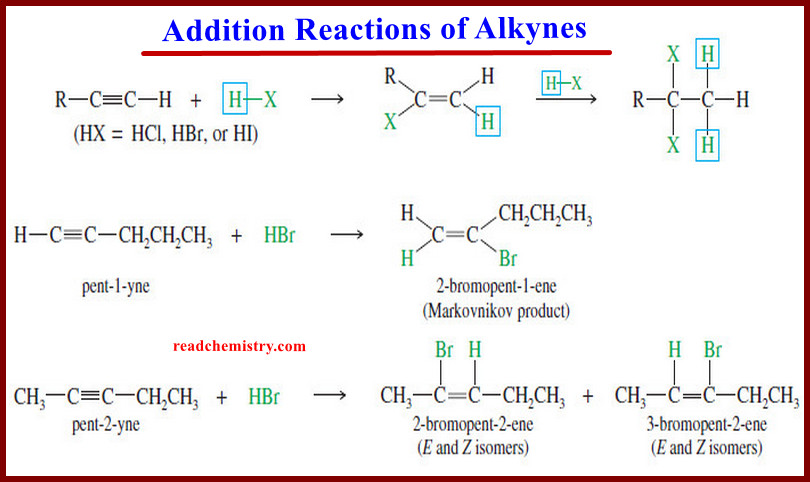
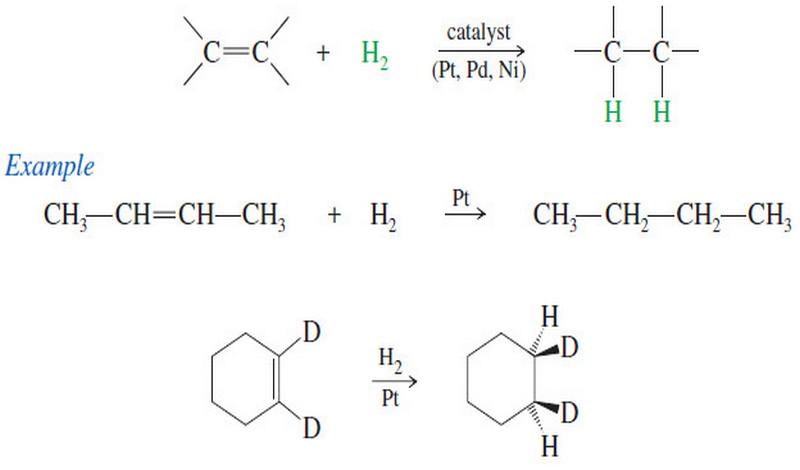
Addition Reactions of Alkynes
Addition Reactions of Alkynes – Many of the reactions of alkynes are similar to the corresponding reactions of alkenes because…
Read More » -
Organic Chemistry
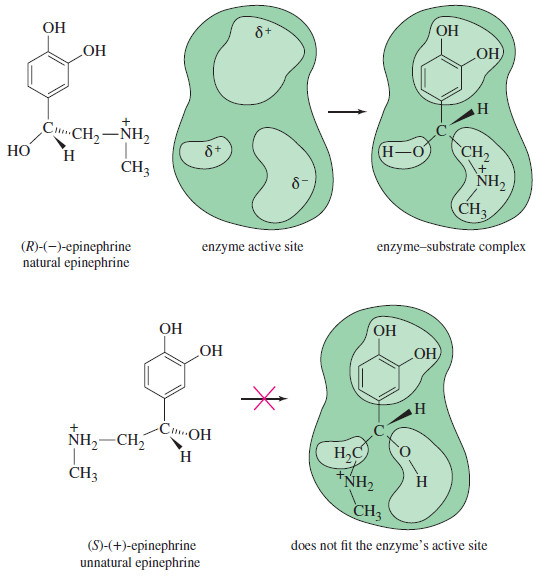
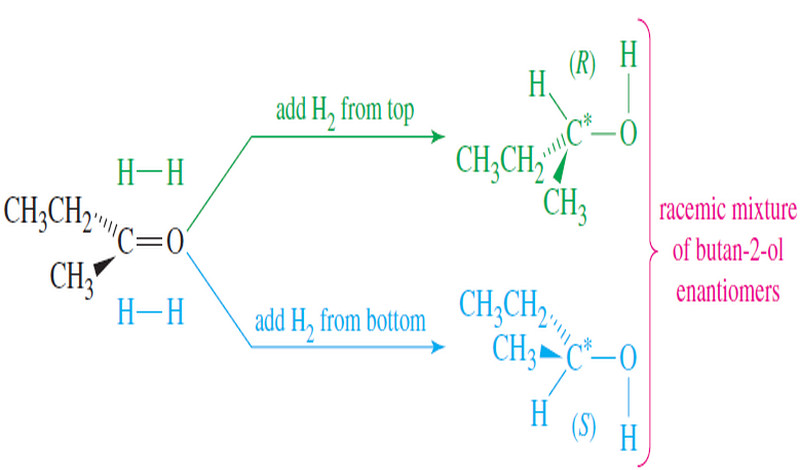
Biological Discrimination of Enantiomers
Biological Discrimination of Enantiomers – If the direction of rotation of polarized light were the only difference between enantiomers, one…
Read More » -
General Chemistry
Molecular Orbitals for Homonuclear Diatomic Molecules
Molecular Orbitals for Homonuclear Diatomic Molecules – In the previous subject, we talk about but electronic structures and bonding properties…
Read More » -
Organic Chemistry
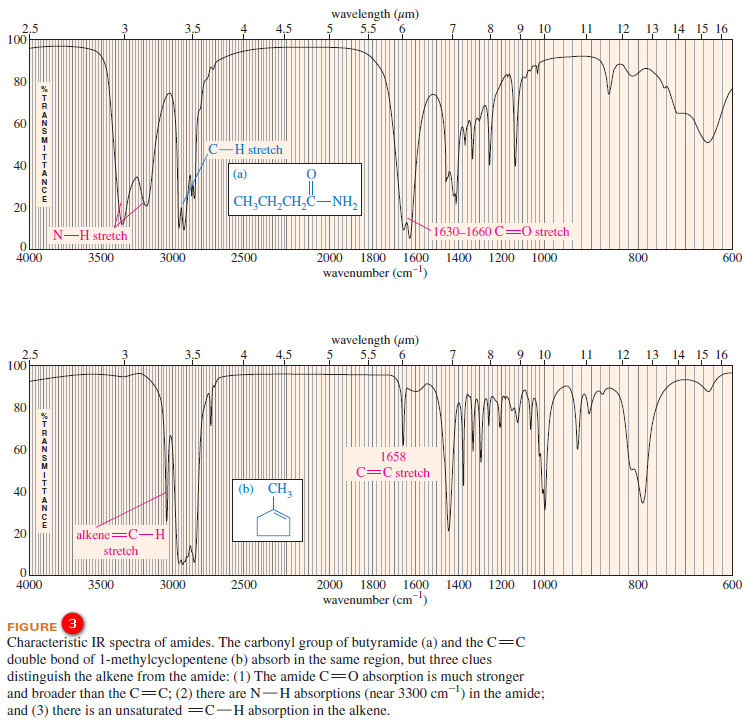
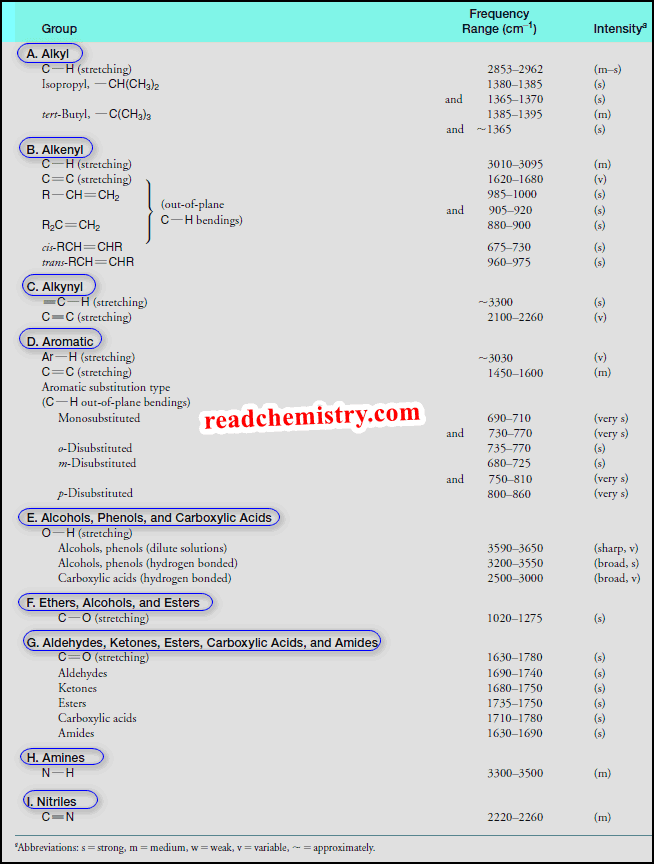
Characteristic Absorptions of Carbonyl Compounds
– In this subject, we will talk about Characteristic Absorptions of Carbonyl Compounds such as Ketones, Aldehydes, Amines, and Acids.…
Read More » -
Physical Chemistry
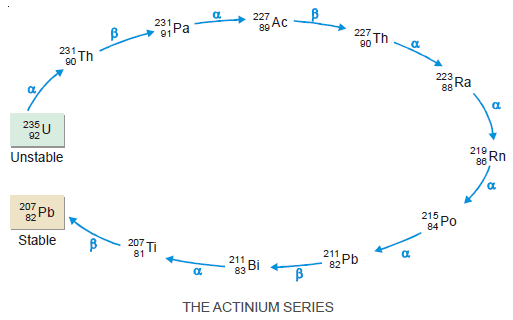
Radioactive Disintegration Series: Definition, Examples
– In this subject, we will discuss the Radioactive Disintegration Series: Definition, Examples Radioactivity – Several elements such as uranium…
Read More »
-
Organic Chemistry
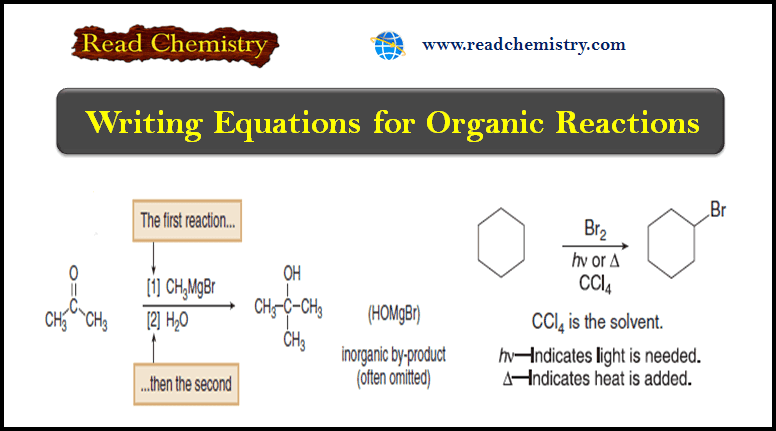
Writing Equations for Organic Reactions
– In this subject, we will discuss Writing Equations for Organic Reactions. Writing Equations for…
Read More » -
-
-
-
-
-
-
-
-
-
-
Physical Chemistry
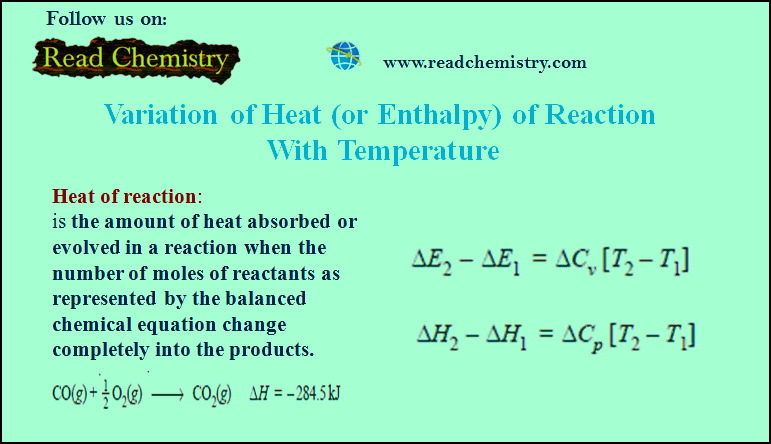
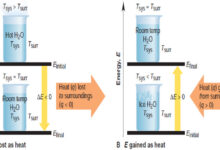
Variation of heat of reaction with temperature
– In this subject, the Variation of heat of reaction with temperature will be discussed.…
Read More » -
-
-
-
-
-
-
-
-
-
-
General Chemistry
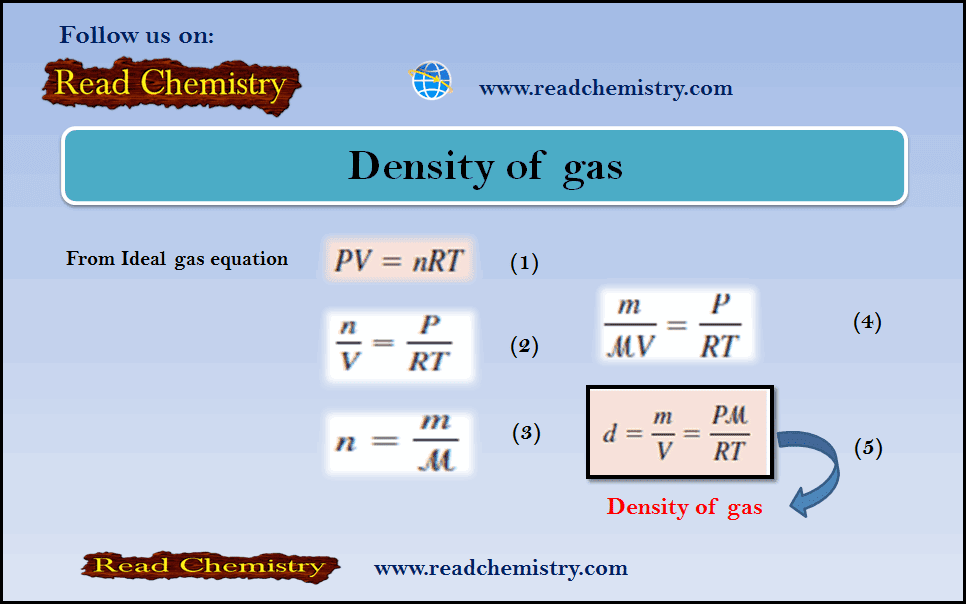
Density of gas: Definition, Equation, Solved Examples
– In this subject, we will discuss the Density of gas (Definition, Equation, Solved Examples)…
Read More » -
-
-
-
-
-
-
-
-
-
-
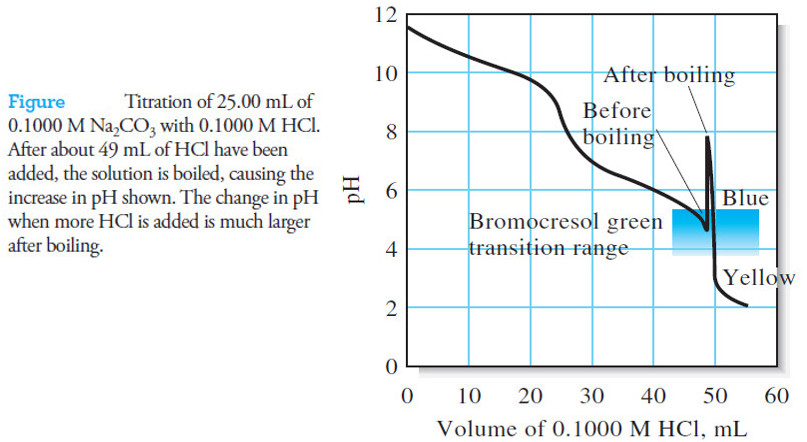
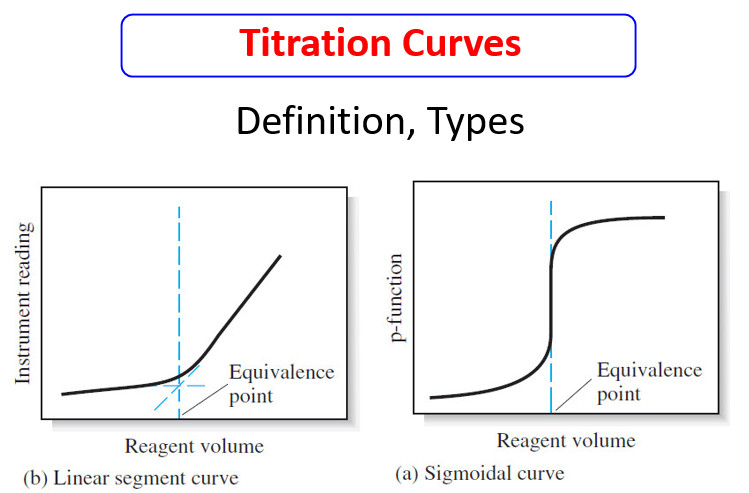
Analytical Chemistry
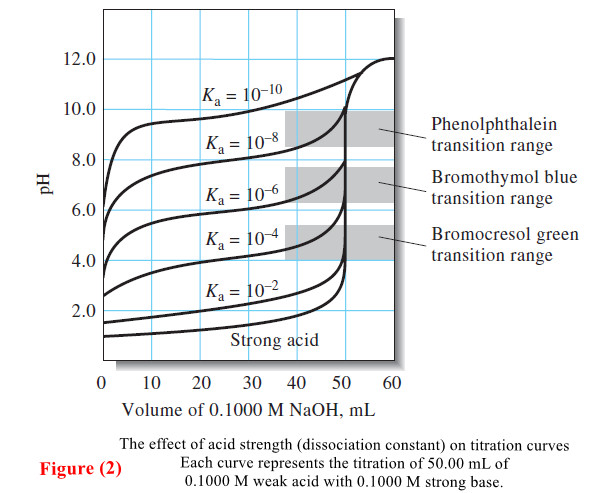
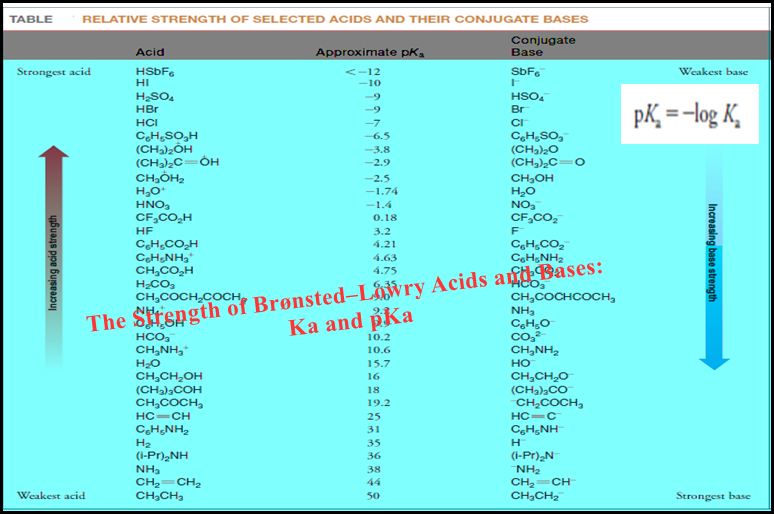
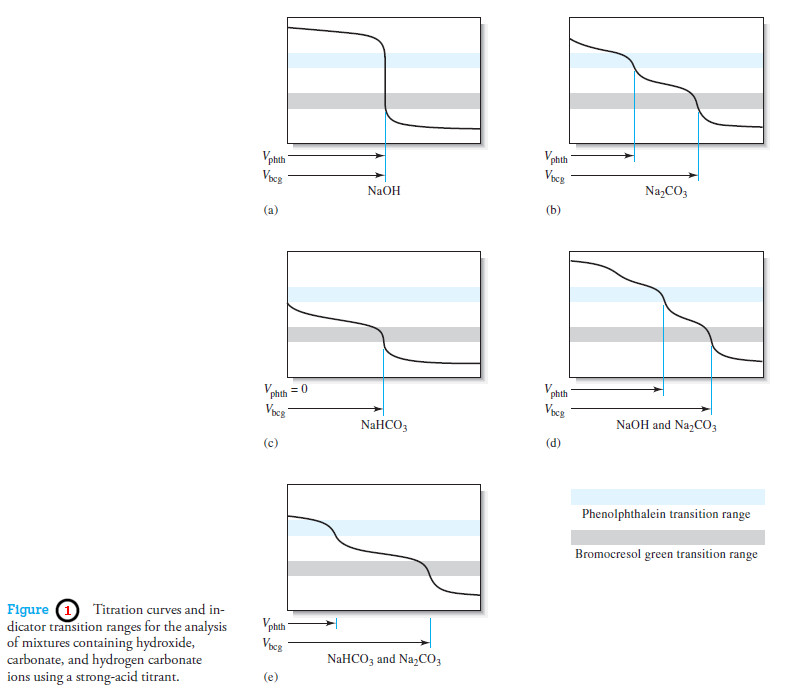
Titration Curves for Weak Acids
Titration Curves for Weak Acids – Four distinctly different types of calculations are needed to…
Read More » -
-
-
-
-
-
-
-
-
-
-
Online MCQ
First law of thermodynamics – MCQ online test
Online MCQ test on First law of thermodynamics – In this topic we offer you,…
Read More » -
-
-
-
-
-
-
-
Free book
Physical Chemistry book , 3rd edition by Robert G. Mortimer
– In this subject, we will discuss free download of Physical Chemistry book, 3rd edition…
Read More » -
-
-
-
-