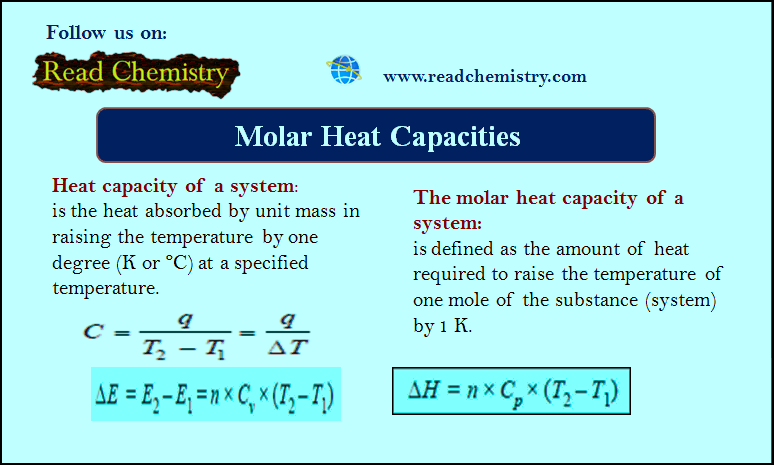
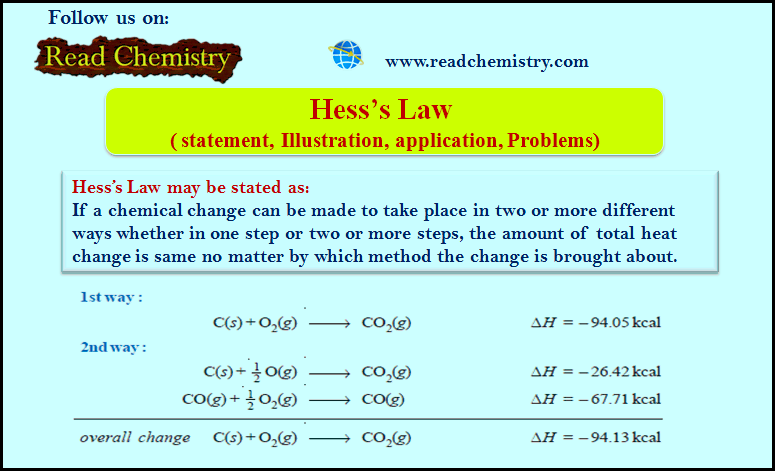
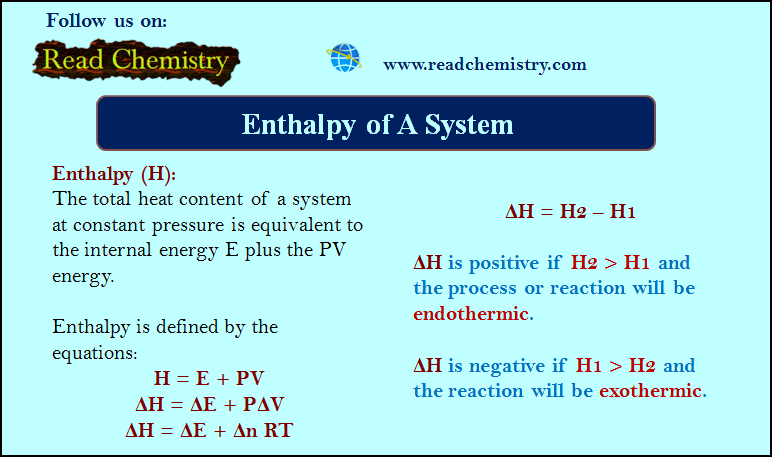
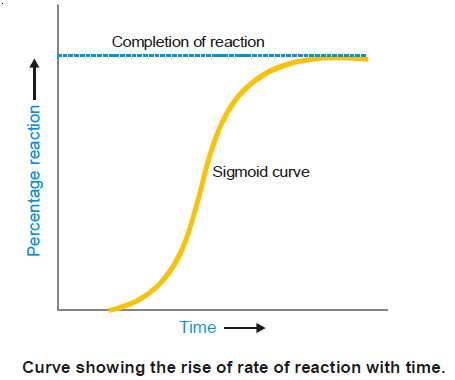
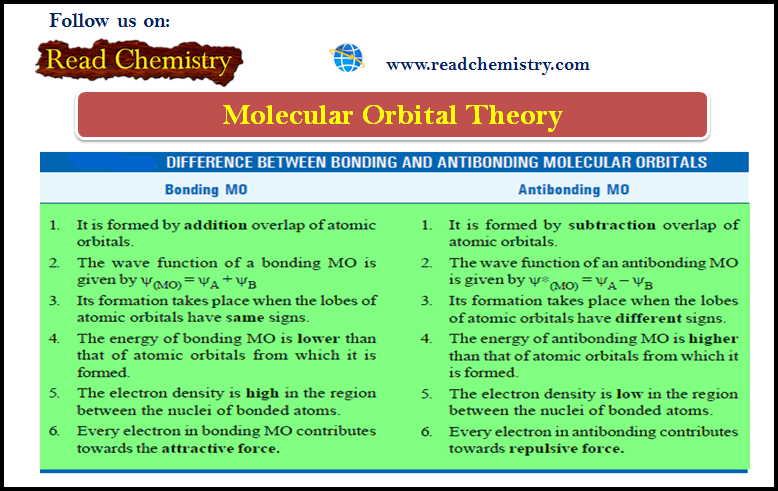
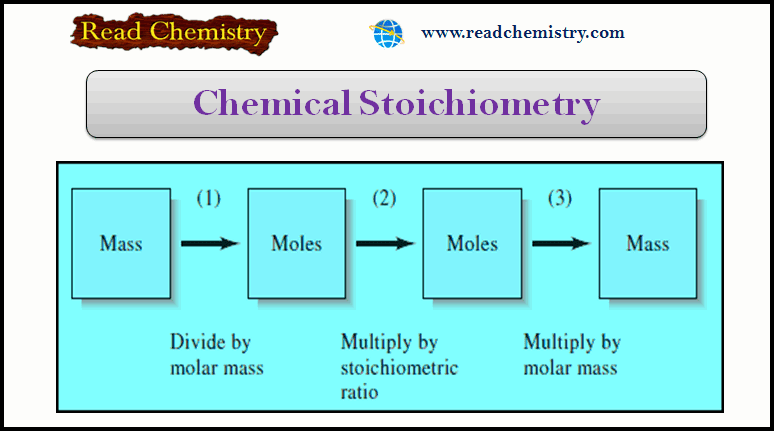
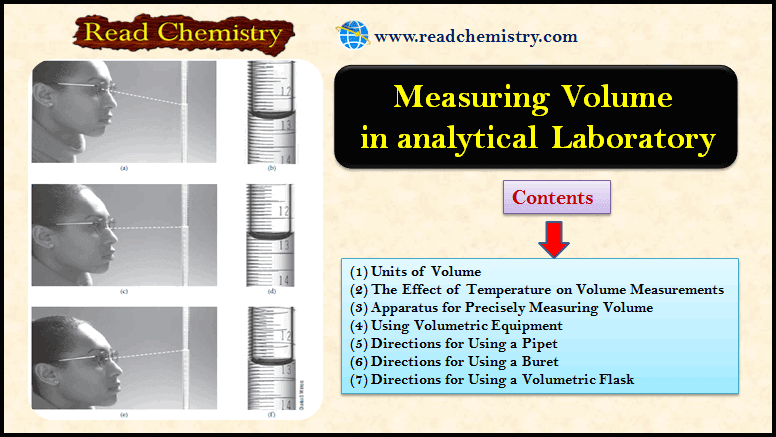
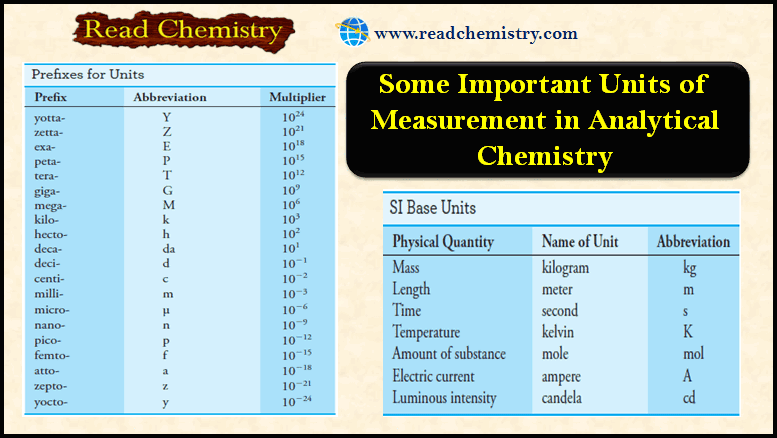
Popular Posts
-
General Chemistry
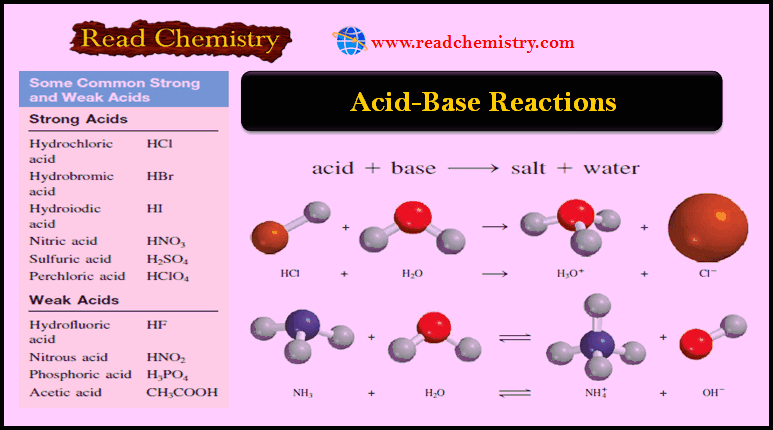
Acid-Base Reactions: Definition, Examples, and Uses
– In this subject, we will discuss the Acid-Base Reactions: Definition, Examples, and Uses – Acids and bases are as…
Read More » -
Organic Chemistry
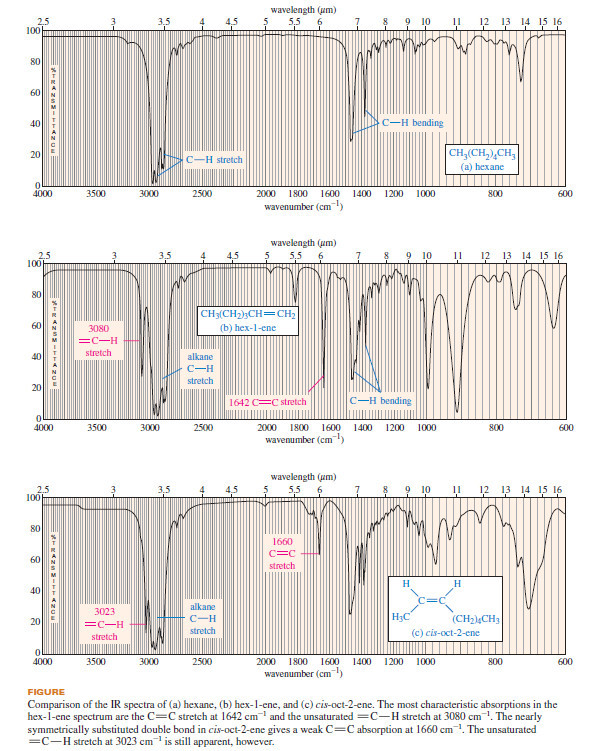
Hydrocarbons: Infrared Spectroscopy of Hydrocarbons
Infrared Spectroscopy of Hydrocarbons – Hydrocarbons contain only carbon–carbon bonds and carbon–hydrogen bonds. – An infrared spectrum does not provide…
Read More » -
Physical Chemistry
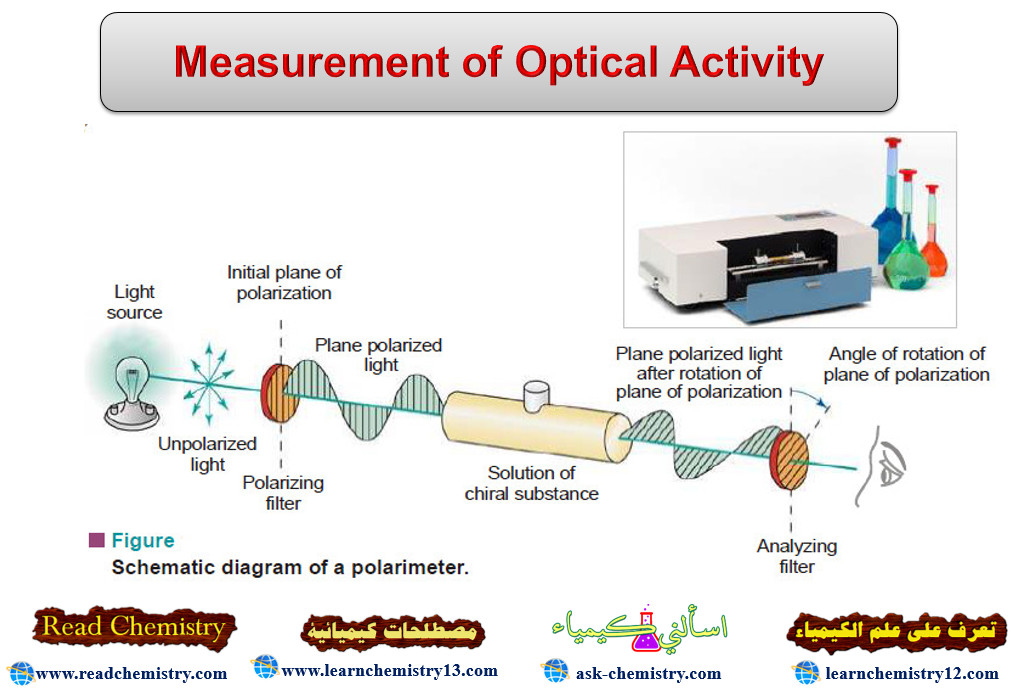
Measurement of Optical Activity
Optical Activity – Optical activity is one of imortant physcial properties of liqiuds – A beam of ordinary light consists…
Read More » -
Organic Chemistry
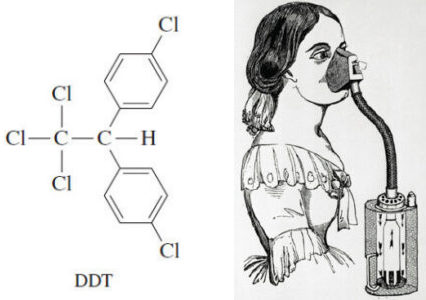
Common Uses of Alkyl Halides
Alkyl halides as Solvents – Alkyl halides are used primarily as industrial and household solvents. – Carbon tetrachloride (CCl4) was…
Read More » -
Analytical Chemistry
Acid-Base Theories: Arrhenius, Lewis, and Bronsted-Lowry Theory
– In this subject, we will discuss Acid-Base Theories: Arrhenius, Lewis, and Bronsted-Lowry Theory Acid-base Theories – Several acid–base theories…
Read More » -
Organic Chemistry
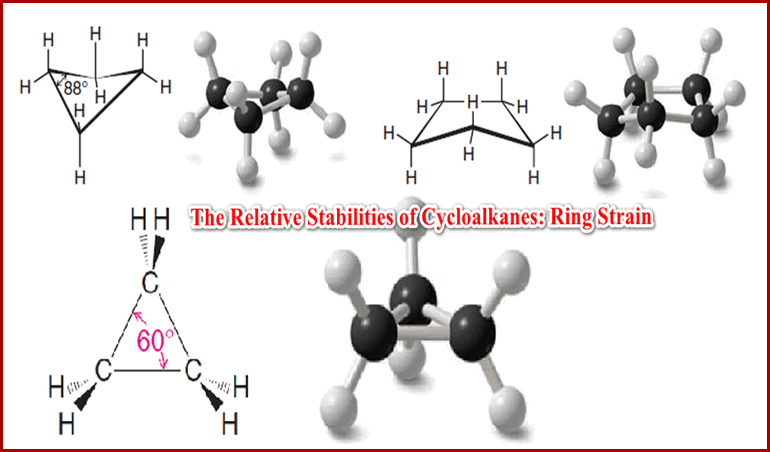
The Relative Stabilities of Cycloalkanes: Ring Strain
The Relative Stabilities of Cycloalkanes: Ring Strain ** Cycloalkanes do not all have the same relative stability. Experiments have…
Read More »
-
Organic Chemistry
Reactions of Alkyl Halides
In this subject we will discuss the Reactions of Alkyl Halides with chemical equations and…
Read More » -
-
-
-
-
-
-
-
-
-
-
Physical Chemistry
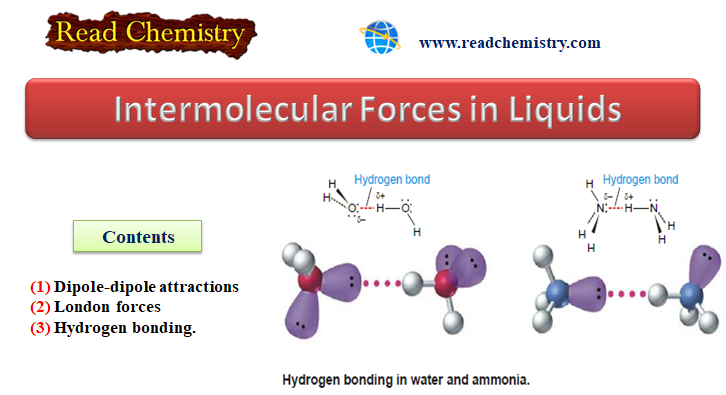
Intermolecular Forces in Liquids
Intermolecular Forces in Liquids – Intermolecular forces in liquids are collectively called van der Waals…
Read More » -
-
-
-
-
-
-
-
-
-
-
General Chemistry
Matter and Energy
Definition of Matter and Energy What is Matter? – Matter is anything that has mass…
Read More » -
-
-
-
-
-
-
-
-
-
-
Analytical Chemistry
Concentration of Solutions: Definitions, Formulas, Solved Problems
– In this subject, we will discuss the Concentration of Solutions Concentration of Solutions (Definitions,…
Read More » -
-
-
-
-
-
-
-
-
-
-
Online MCQ
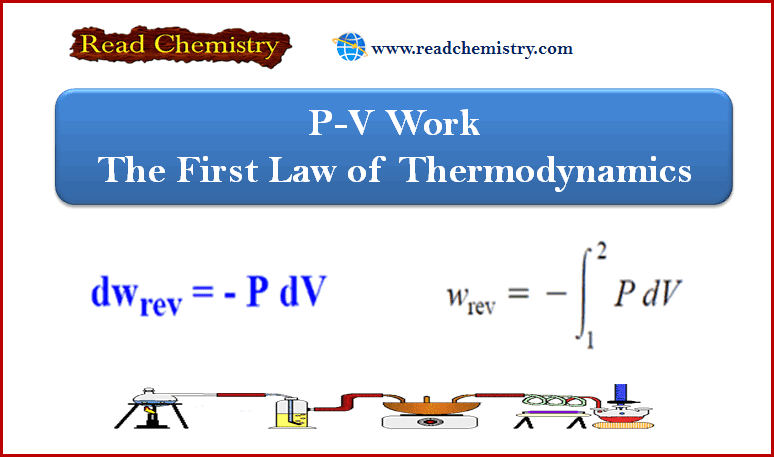
First law of thermodynamics – MCQ online test
Online MCQ test on First law of thermodynamics – In this topic we offer you,…
Read More » -
-
-
-
-
-
-
-
Free book
Physical Chemistry book , 3rd edition by Robert G. Mortimer
– In this subject, we will discuss free download of Physical Chemistry book, 3rd edition…
Read More » -
-
-
-
-