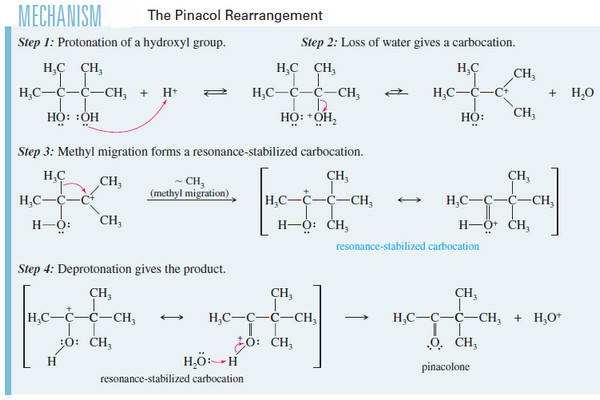
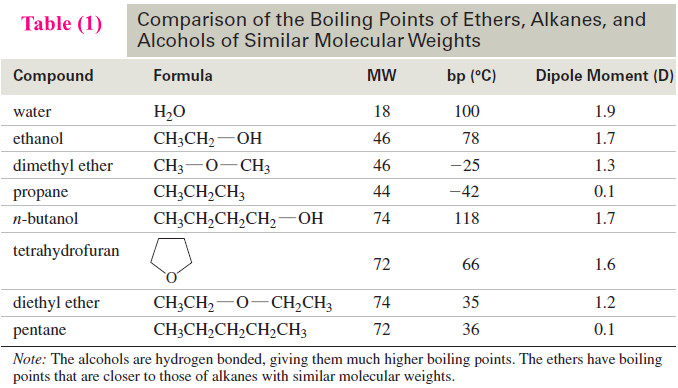
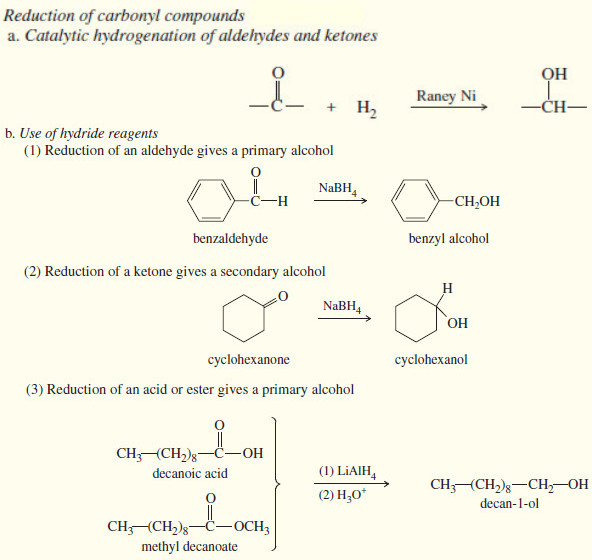
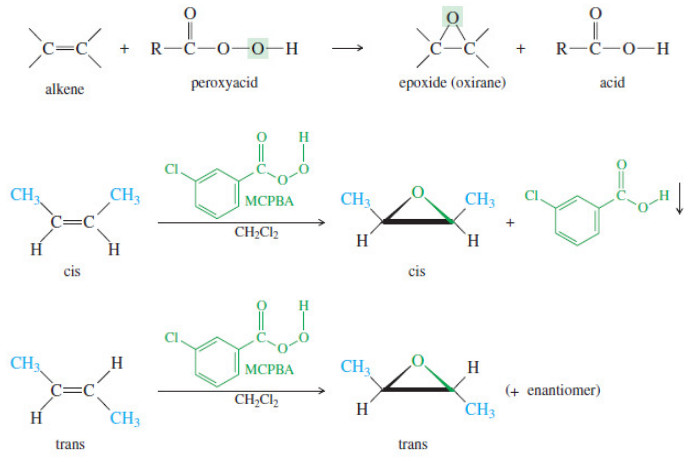
Popular Posts
-
Organic Chemistry
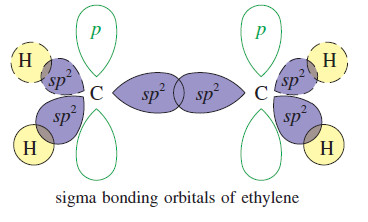
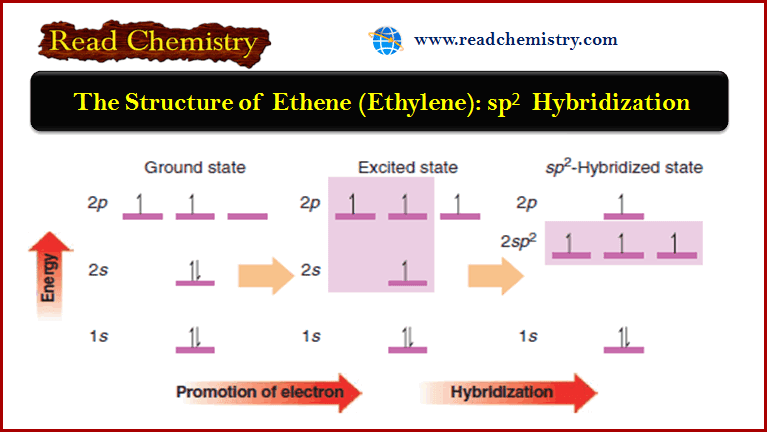
The Orbital Description of the Alkene Double Bond
The Orbital Description of the Alkene Double Bond – In a Lewis structure, the double bond of an alkene is…
Read More » -
General Chemistry
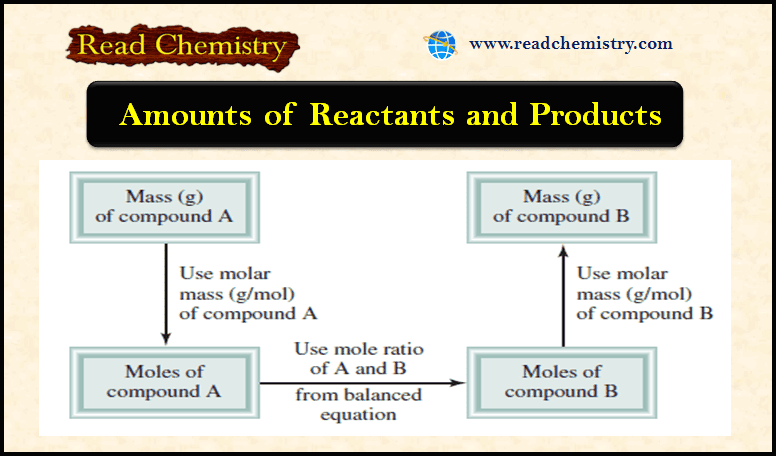
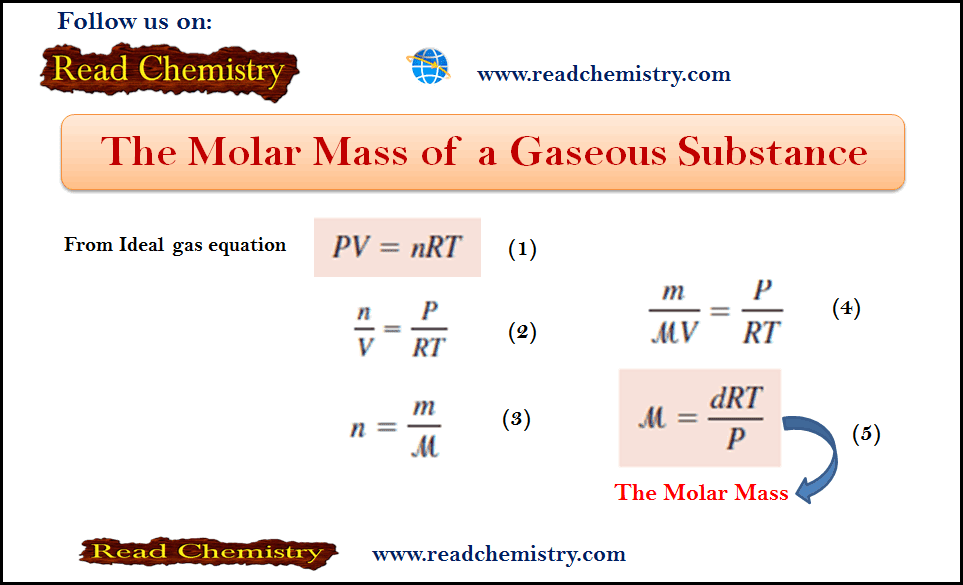
Amounts of Reactants and Products
– In this subject, we will discuss Amounts of Reactants and Products Amounts of Reactants and Products – A basic…
Read More » -
Physical Chemistry
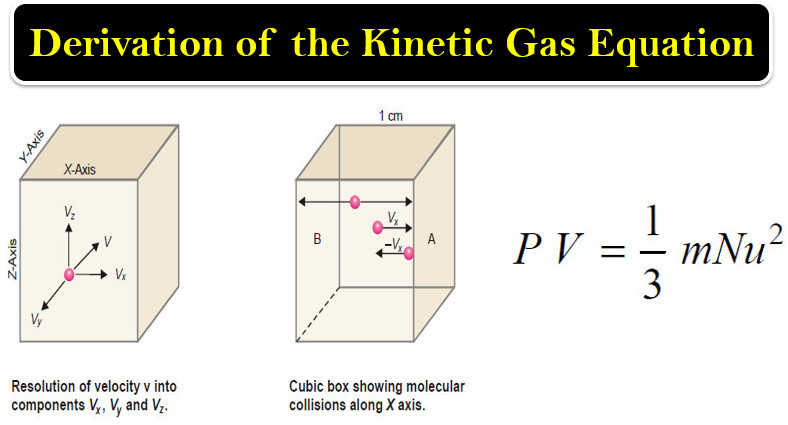
Derivation of the Kinetic Gas Equation
– In this topic, we will talk about Derivation of the Kinetic Gas Equation. Derivation of the Kinetic Gas Equation…
Read More » -
General Chemistry
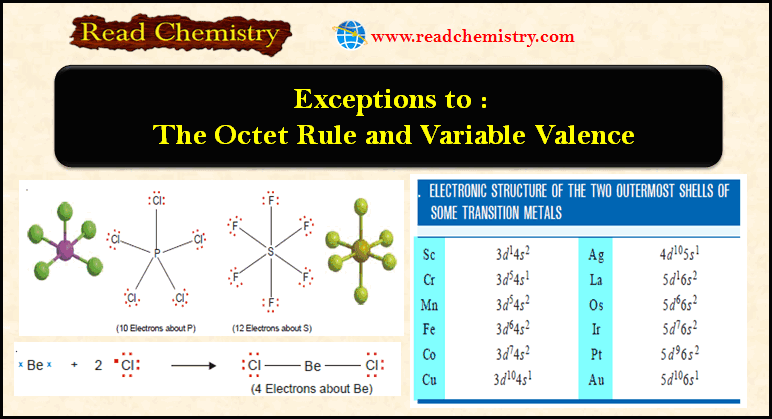
Exceptions to the Octet Rule and Variable Valence
Exceptions to the octet rule – For a time it was believed that all compounds obeyed the Octet rule or…
Read More » -
Organic Chemistry
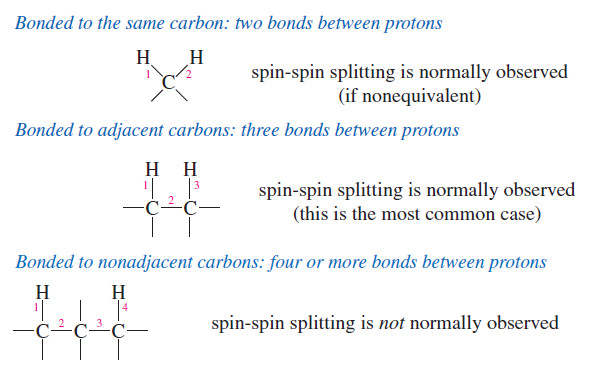
Spin-Spin Splitting in ¹H NMR Spectra
– In this topic, we will discuss The Spin-Spin Splitting in ¹H NMR Spectra. Theory of Spin-Spin Splitting – A…
Read More » -
Organic Chemistry
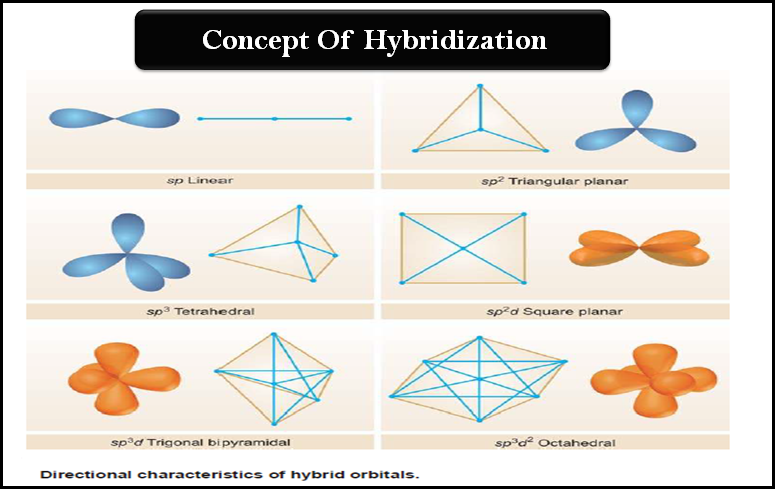
Hybridization: Definition, Types, Rules, Examples
– In this subject, we will discuss the Hybridization: Definition, Types, Rules, and Examples – While the formation of simple…
Read More »
-
Organic Chemistry
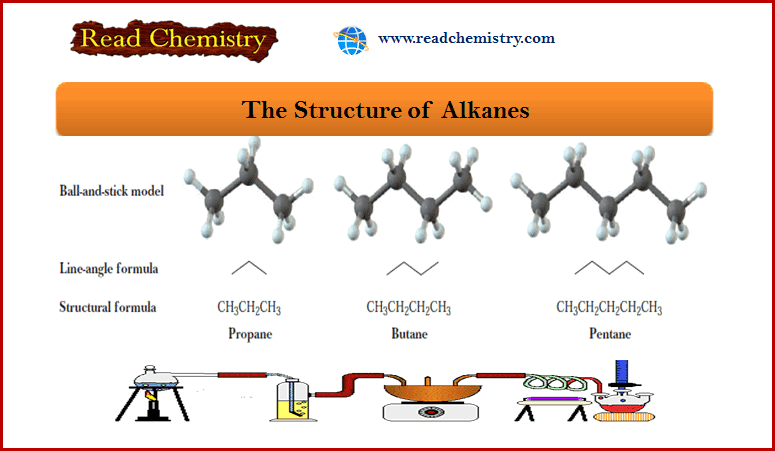
Alkanes: Definition, Formula, Structure, List, Examples
What is Alkanes? – Alkanes are saturated hydrocarbons; that is, they contain only carbon-carbon single…
Read More » -
-
-
-
-
-
-
-
-
-
-
Physical Chemistry
Applications of distribution law
Applications of distribution law – There are numerous applications of distribution law in the laboratory…
Read More » -
-
-
-
-
-
-
-
-
-
-
General Chemistry
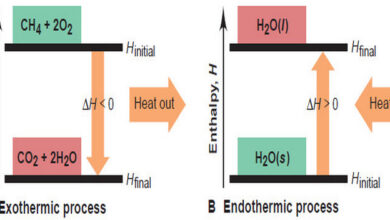
Enthalpy: Heats of Reaction and Chemical Change
In this subject, we will discuss the Enthalpy: Heats of Reaction and Chemical Change. Enthalpy:…
Read More » -
-
-
-
-
-
-
-
-
-
-
Analytical Chemistry
Applications of Neutralization Titrations
– In this topic, we will discuss The Applications of Neutralization Titrations. Typical Applications of…
Read More » -
-
-
-
-
-
-
-
-
-
-
Online MCQ
First law of thermodynamics – MCQ online test
Online MCQ test on First law of thermodynamics – In this topic we offer you,…
Read More » -
-
-
-
-
-
-
-
Free book
Physical Chemistry book , 3rd edition by Robert G. Mortimer
– In this subject, we will discuss free download of Physical Chemistry book, 3rd edition…
Read More » -
-
-
-
-